- Get link
- X
- Other Apps
- Get link
- X
- Other Apps
Five years ago the largest medical device recall was nearly twice the size of that in 2017 caused by a single sterility-compromised product that resulted in 102 million devices being recalled. The information contained herein is a compilation from different sources.
 Medical Device Recalls Emma International
Medical Device Recalls Emma International
If you wish to find information on a recall or a correction or removal action that has not yet been classified you can search the Medical Device Recalls Database.

Medical device recalls. ADVIA Centaur Enhanced Human Immunodeficiency Virus 1O2 Test Kits. Medtronic Inc Cardiac Rhythm and Heart Failure CRHF Z-2506-2020 - Patient Connector Model Number 24967. Build your study within hours.
Z-2505-2020 - CareLink SmartSync Device Manager Model Number 24970A. 4095 million medical device units were recalled in 2016-2017 averaging 5819 million units per quarter. When a product recall occurs the communication between the device company and the hospital needs to be prompt well structured and involve all the relevant stakeholders.
Castor ranks amongst the top 5 EDCs for shortest build times. Since January 2017 it may also include correction or removal actions initiated by a. This database contains Medical Device Recalls classified since November 2002.
Class I medical device recalls are the most serious and urgent under FDA classification Medical device failures and malfunctions invariably harm peoples wellbeing due to the fact they are often deployed on the frontline of patient care and in users homes. Explore more than 120000 Recalls Safety Alerts and Field Safety Notices of medical devices and their connections with their manufacturers. Class 2 Medicines Recall.
By comparison the largest medical device recall in 2017 was 54 million units also caused by sterility problems. Build your study within hours. QUADROX-i Oxygenators with Integrated Arterial Filter.
This statistic shows the distribution of major causes for medical device recalls in the United States as of second quarter of 2019 based on. In August 2017 medical device company Abbott stated that it was voluntarily recalling almost 500000 of its pacemakers in the US due to a cyber-security flaw. The cyber-security flaw meant that the companys pacemakers could be hacked into with the perpetrator being able to speed-up or slow down the device.
Medical Devices Recalls This section has been designed to provide information about medical devices that have been recalled from markets by manufacturers. Hospitals are generally notified of a recall via a letter sent in the mail from the manufacturer. Advertentie Improve your research process data quality.
International Medical Devices Database By the International Consortium of Investigative Journalists. Maquet Cardiopulmonary AG A Getinge Group Co. Ennogen Pharma Limited Trimethoprim 200mg Tablets PL 401470083 EL 21A10 Ennogen Pharma Limited are recalling an affected batch of Trimethoprim 200mg Tablets as a.
Siemens Healthcare Diagnostics GmbH. Date Recall Posted Recalling Firm. In 2018 and the first two quarters of 2019 that average shot up to 9933 million units recalled per quarter or 59598 million in just 18 months time.
Advertentie Improve your research process data quality. Castor ranks amongst the top 5 EDCs for shortest build times. A recall in respect of a medical device that has been sold means any action taken by the manufacturer importer or distributor of the device to recall or correct the device or to notify its owners and users of its defectiveness or potential defectiveness after.
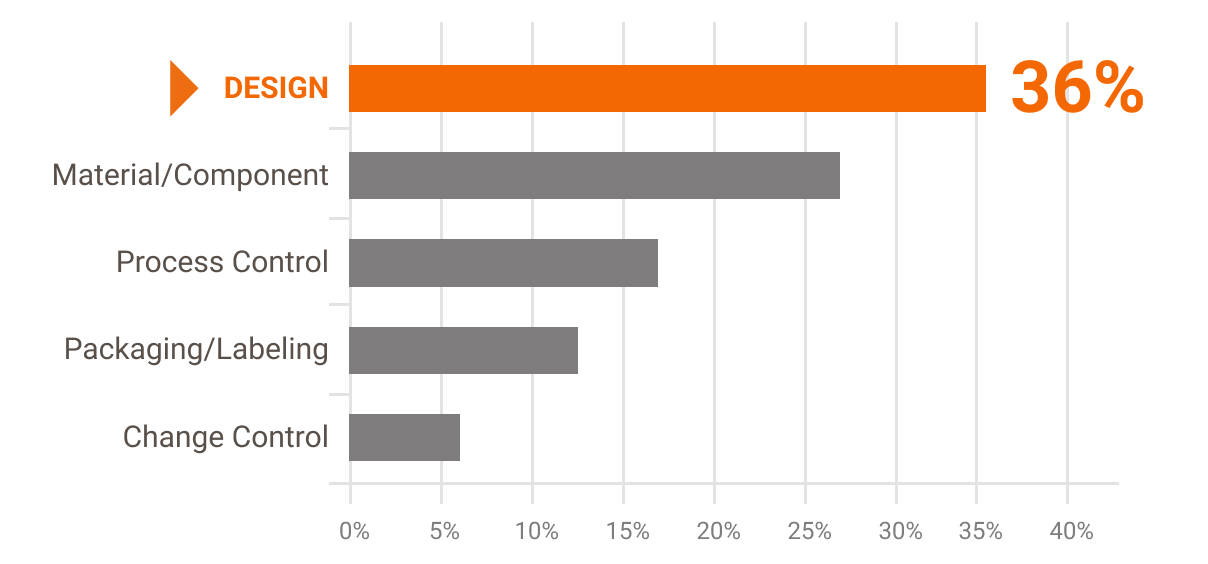 Focus On Usability Can Limit Medical Device Recalls By Stephanie Van Ness Medium
Focus On Usability Can Limit Medical Device Recalls By Stephanie Van Ness Medium
 Protecting The Patient With Safety Recall Data
Protecting The Patient With Safety Recall Data
 Trends In Medical Device Recalls Medtech Intelligence
Trends In Medical Device Recalls Medtech Intelligence
 Fda Medical Device Recalls 2009 2017 Download Scientific Diagram
Fda Medical Device Recalls 2009 2017 Download Scientific Diagram
 Report Medical Device Recalls Down In Q4 Of 2017 Medical Design And Outsourcing
Report Medical Device Recalls Down In Q4 Of 2017 Medical Design And Outsourcing
 Medical Device Recalls Spike Again Updated Mddionline Com
Medical Device Recalls Spike Again Updated Mddionline Com
 Fda Medical Device Recalls Now And Then
Fda Medical Device Recalls Now And Then
Medical Device Recalls Hit 2 Year Low In Q2 Massdevice
 Trends In Medical Device Recalls Medtech Intelligence
Trends In Medical Device Recalls Medtech Intelligence
 Trends In Medical Device Recalls Medtech Intelligence
Trends In Medical Device Recalls Medtech Intelligence
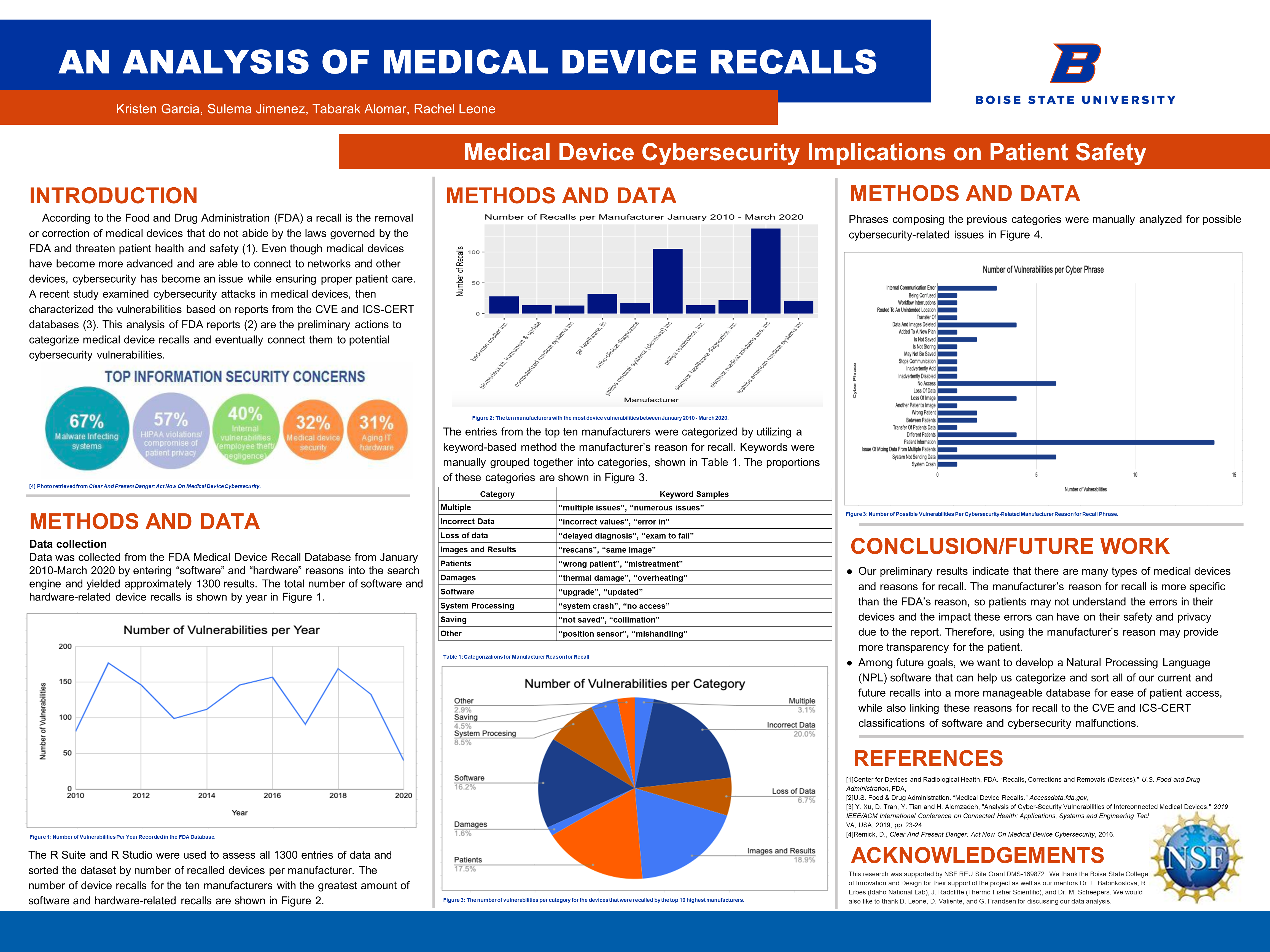 118 An Analysis Of Medical Device Recalls Undergraduate Research
118 An Analysis Of Medical Device Recalls Undergraduate Research
 Is Software The Weak Link In Medical Device Safety Mddionline Com
Is Software The Weak Link In Medical Device Safety Mddionline Com
 Medical Device Recalls Per Quarter U S 2015 2019 Statista
Medical Device Recalls Per Quarter U S 2015 2019 Statista
 Medical Device Recalls Were Way Up In 2019 Mddionline Com
Medical Device Recalls Were Way Up In 2019 Mddionline Com
Comments
Post a Comment