- Get link
- X
- Other Apps
- Get link
- X
- Other Apps
A public health risk. It is legal in many countries including the United States based on the premise that regulatory agencies do not have the authority to control the practice of medicine.
What Does Off Label Drug Mean Youtube
Promotion of any drug or medical device for a purpose not included on its FDA cleared label.

What does off label mean. Off label Adverb Use of a prescription medication by a physician to treat a condition other than that for which the drug was approved by the Food and Drug Administration FDA. What is meant by off-label and unlicensed use. Regulated by state medical boards as part of the practice of medicine.
The Food and Drug Administration FDA approve drugs for certain health conditions. Christie Long DVM Veterinarian Certified Veterinary Acupuncturist. Ask Christie Long DVM Now.
What does off-label mean. Doctors may have found that the medicine works very. This practice is known.
The American Heritage Medical. Some examples of off-label uses are. The medication Ketamine is approved by the FDA to be used as an anesthetic.
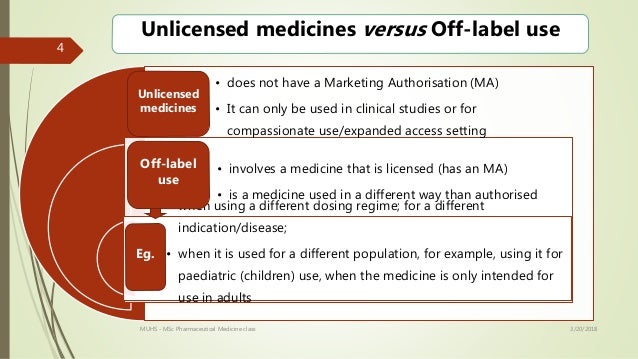
This term can mean that the drug is. Technically off-label prescribing means the prescribing of medications or devices for indications or population subgroups that regulatory agencies have not officially approved. Off-label means the medication is being used in a manner not specified in the FDAs approved packaging label or insert.
Using a medicine for a different illness to that stated in the licence. Of relating to or being a drug used to treat a condition for which it has not been officially approved. Information and translations of off-label in the most comprehensive dictionary definitions resource on the web.
Of or relating to a drug prescribed to treat a condition for which it has not been approved by the Food and Drug Administration. Small animal practitioner with 10 years experience in medicine surgery and acupuncture. What does Off label mean.
Wiktionary 000 0 votes Rate this definition. In the United States the regulations of the Food and Drug Administration FDA permit physicians to prescribe approved medications for other than their intended indications. Off-label use means that the medicine is being used in a way that is different to that described in the licence.
Used for a disease or medical condition that it. What Does Off-Label Mean. Committed by pharmaceutical and medical device manufacturers.
But what does it really mean. Every prescription drug marketed in the US. Unfair to patients and physicians.
When a doctor prescribes a drug off-label they are prescribing it for a different condition or at a different. Used to describe a medical drug that is used for a different purpose than the one it was originally. It is not FDA approved to be used in the treatment of depression PTSD anxiety OCD postpartum depression or chronic pain.
Unapproved use of an approved drug is often called off-label use.
Off Label Use Of Drugs Devices
 30 What Does Off Label Mean Labels Database 2020
30 What Does Off Label Mean Labels Database 2020
 30 What Does Off Label Mean Labels Database 2020
30 What Does Off Label Mean Labels Database 2020
 Summary Of Definitions Download Table
Summary Of Definitions Download Table
Off Label Drug Use Shouldn T Mean No Access
 Off Label Or Off Limits Should You Use A Drug For An Unapproved Use
Off Label Or Off Limits Should You Use A Drug For An Unapproved Use
33 What Does Off Label Mean Labels For Your Ideas




Comments
Post a Comment