- Get link
- X
- Other Apps
- Get link
- X
- Other Apps
The FDA had never granted an EUA for a vaccine for the entire population before and the standards for an EUA were seen by some as lower than for full approval she said. The Pfizer COVID-19 vaccine is the only one that has emergency approval for 12-year-olds and older -- the FDA approved the Pfizer vaccine for kids between the ages of 12 and 15 earlier this week.
 Us Fda Experts Approve Biontech Pfizer Covid Vaccine News Dw 11 12 2020
Us Fda Experts Approve Biontech Pfizer Covid Vaccine News Dw 11 12 2020
And while the FDA has authorized the Pfizer Moderna and Johnson Johnson vaccines and is expected to also approve each one in turn for many people the difference between authorized and approved has been confusing.

Vaccines approved by fda. Clinical trials are evaluating investigational COVID-19 vaccines in tens of thousands of study participants to generate the scientific data and other information needed by FDA to determine. Currently no coronavirus vaccine is fully approved by the FDA but three were given emergency use authorization by the agency Published April 14 2021 Updated on April 14 2021 at 145 pm NBC. Vaccine makers will need to apply to the FDA for full approval to continue use after the pandemic.
As long as no serious harms from the vaccines are discovered theyll. No none of the COVID-19 vaccines have received FDA approval yet however Pfizer Moderna and Johnson Johnson were all granted emergency use authorization EUA. Pfizer hopes to join the ranks of the flu shot measles mumps rubella shot and other vaccines that are fully FDA approved and licensed.
Pfizer and BioNTech asked the Food and Drug Administration Friday for full approval of the companies Covid-19 vaccine. Vaccines for Use During Pregnancy to Protect Young Infants from Disease - FDA Update. Anthony Fauci director of the National Institute of Allergy and Infectious Diseases told CNNs Jim Sciutto on Wednesday he hopes Covid-19 vaccines will receive full FDA approval very soon.
- Pfizers application for full FDA approval of its COVID-19 vaccines could impact vaccine hesitancy and businesses that may consider vaccine mandates. The FDA granted an EUA for the Pfizer and BioNTech vaccine and its likely to do the same for the Moderna vaccine this week. On Tuesday the company Pfizer said that it plans to file for full FDA approval of its COVID-19 vaccine in the US.
Alex Tin contributed reporting. Pfizer and its vaccine partner BioNTech have started an application to request the Food and Drug Administrations approval for its COVID-19 vaccine. By the end of.
Pfizer has also asked the FDA for full approval of its vaccine which means it would no longer be distributed under an emergency use authorization. 91 Zeilen COVID-19 Vaccines FDA COVID-19 Vaccines April 23 2021. If approved it would be the first Covid-19 vaccine in the United States to.
The FDA amended the emergency use authorization of the Johnson Johnson Janssen COVID-19 vaccine to include information about a very rare and serious type of blood clot in. April 23 2021. Claims that COVID-19 vaccines are.
Pfizer is the first coronavirus vaccine maker in. On December 11 2020 the US. According to the FDA An Emergency Use Authorization EUA is a mechanism to facilitate the availability and use of medical countermeasures including vaccines.
30 Zeilen Pfizer-BioNTech COVID-19 Vaccine.
 Pfizer Covid 19 Vaccine Fda Report Released Ahead Of Emergency Use Meeting
Pfizer Covid 19 Vaccine Fda Report Released Ahead Of Emergency Use Meeting
Fda Sets Bar For Covid 19 Vaccine Approval At 50 Effectiveness Biospace
 What S The Difference Between Fda Emergency Use Authorization And Fda Approval Unc Health Covid 19 Vaccine Hub
What S The Difference Between Fda Emergency Use Authorization And Fda Approval Unc Health Covid 19 Vaccine Hub
 Fda Approves Pfizer Biontech Coronavirus Vaccine For Emergency Use In Us Coronavirus The Guardian
Fda Approves Pfizer Biontech Coronavirus Vaccine For Emergency Use In Us Coronavirus The Guardian
 Fda Issues Emergency Use Authorization For Third Covid 19 Vaccine Fda
Fda Issues Emergency Use Authorization For Third Covid 19 Vaccine Fda
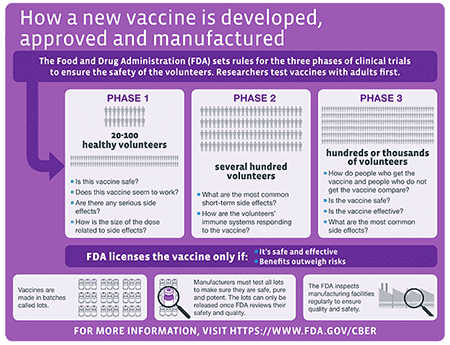 Ensuring The Safety Of Vaccines In The United States Cdc
Ensuring The Safety Of Vaccines In The United States Cdc
 J J S Single Shot Covid 19 Vaccine Approved By Fda Bioprocess Insiderbioprocess International
J J S Single Shot Covid 19 Vaccine Approved By Fda Bioprocess Insiderbioprocess International
 Pfizer Biontech Vaccine Starts Working 10 Days After First Dose Says Fda Financial Times
Pfizer Biontech Vaccine Starts Working 10 Days After First Dose Says Fda Financial Times
Fda Approves Johnson Johnson Vaccine For Use In Us Voice Of America English
 Learn More About Covid 19 Vaccines From The Fda Fda
Learn More About Covid 19 Vaccines From The Fda Fda
 Fda Approves Emergency Use Of A 2nd Covid 19 Vaccine
Fda Approves Emergency Use Of A 2nd Covid 19 Vaccine
 Moderna S Covid 19 Vaccine Becomes Second To Receive Fda Approval Financial Times
Moderna S Covid 19 Vaccine Becomes Second To Receive Fda Approval Financial Times

Comments
Post a Comment