- Get link
- X
- Other Apps
- Get link
- X
- Other Apps
While the drugs approval in Europe and Japan bodes well for Gilead its efforts to obtain an. The California-based Gilead Sciences said Veklury is approved for people at least 12 years old and weighing at least 88 pounds who need hospitalization for their virus infection.
 Gilead S Biktarvy Wins Megablockbuster Hiv Nod And Rival Glaxosmithkline Strikes Back Fiercepharma
Gilead S Biktarvy Wins Megablockbuster Hiv Nod And Rival Glaxosmithkline Strikes Back Fiercepharma
Is a research-based biopharmaceutical company focused on the discovery development and commercialization of innovative medicines.

Gilead approved drugs. The company will receive a milestone payment of 75 million from Gilead related to the approval of the drug in Europe. And tentative approvals of ANDAs and NDAs. Approvals of New Drug Applications NDAs Biologics License Applications BLAs and Abbreviated New Drug Applications ANDAs and supplements to those applications.
Food and Drug Administration for COVID-19. The FDA approved Gilead Sciences antiviral drug remdesivir as a treatment for the coronavirus. GILD today announced that the US.
Gilead GILD obtains full approval for its breast cancer drug Trodelvy in the United States for metastatic triple-negative breast cancer. Gilead Sciences is poised to sell billions of dollars worth of the first COVID-19 treatment approved by US regulators despite mixed evidence on whether or not the drug actually works. There is the possibility of unfavorable results from ongoing and additional clinical trials involving remdesivir and the possibility that Gilead and other parties may be unable to complete one or more of such trials in the currently anticipated timelines or at all.
Food and Drug Administration FDA has approved the antiviral drug Veklury remdesivir for the treatment of patients with COVID-19 requiring hospitalization. Trodelvy a key drug for Gileads ambitions in oncology is now fully approved to treat an aggressive hard-to-treat form of breast cancer. We note that Jyseleca is the first approved drug in the Galapagos portfolio.
We do this through internal research and development as well as through collaborations with academic and industry partners. We now believe the remdesivir clinical trial results are likely to be. Gilead Sciences is acquiring Immunomedics in a 21 billion deal.
Last week Gilead and partner Eisai obtained approval of Jyseleca for this indication in Japan. Original NDA and Original BLA Approvals by Month. Gilead Sciences Inc.
Remdesivir is an investigational drug that has not been approved by the FDA for any use including for the treatment of COVID-19. Gilead Sciences won approval yesterday for a new drug to treat a life-threatening lung disease stealing a march on another company Encysive. All Approvals and Tentative Approvals by Month.
The intravenous drug has helped shorten the recovery time of. GILD announced that the FDA has granted full approval to Trodelvy sacituzumab govitecan-hziy for adult patients with unresectable locally advanced or metastatic. Gilead Sciences Inc.
Food and Drug Administration late Wednesday issued full approval to Gilead Sciences breast cancer drug Trodelvy for certain groups of. As an antiviral drug Veklury works to stop replication of SARS-CoV-2 the virus. Gilead Science Incs antiviral drug remdesivir was granted emergency use authorization by the US.
Gilead is committed to advancing care for patients around the world by bringing forward medicines in areas of unmet medical need. The company also says the drug will complement its approved drugs and those still in the pipeline for treating blood cancers. GILD treatment for coronavirus could be approved for Covid-19 very soon a Piper Sandler analyst says.
FOSTER CITY Calif-- BUSINESS WIRE-- Gilead Sciences Inc.
 F D A Approves Remdesivir As First Drug To Treat Covid 19 The New York Times
F D A Approves Remdesivir As First Drug To Treat Covid 19 The New York Times
 Appeal Lodged Against Decision To Uphold Gilead S Patent On Hepatitis C Drug Msf
Appeal Lodged Against Decision To Uphold Gilead S Patent On Hepatitis C Drug Msf
 Untangling The Trump Administration S Lawsuit Over An Hiv Prevention Drug Science Aaas
Untangling The Trump Administration S Lawsuit Over An Hiv Prevention Drug Science Aaas
 Gilead 2019 Still Gilead Pharmalive
Gilead 2019 Still Gilead Pharmalive
 A Cheat Sheet For The Imminent Data On Gilead S Potential Coronavirus Drug
A Cheat Sheet For The Imminent Data On Gilead S Potential Coronavirus Drug
 Remdesivir Price Still A Puzzle To Be Solved By Gilead Sciences Shots Health News Npr
Remdesivir Price Still A Puzzle To Be Solved By Gilead Sciences Shots Health News Npr
 Gilead Merck Step In To Help India S Drug Manufacturers Fight Surging Covid 19 Outbreak Fiercepharma
Gilead Merck Step In To Help India S Drug Manufacturers Fight Surging Covid 19 Outbreak Fiercepharma

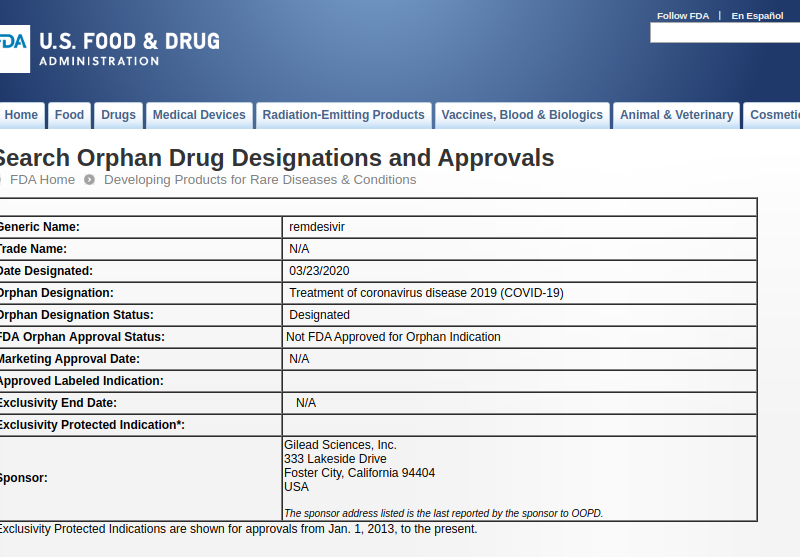 Fda Gives Gilead A Seven Year Regulatory Monopoly For Remdesivir To Treat Covid 19 On Grounds It Is An Orphan Treating A Rare Disease Knowledge Ecology International
Fda Gives Gilead A Seven Year Regulatory Monopoly For Remdesivir To Treat Covid 19 On Grounds It Is An Orphan Treating A Rare Disease Knowledge Ecology International
Gilead Bags Eu Approval For Next Generation Hiv Drug Pmlive

 F D A Approves New H I V Prevention Drug But Not For Everyone The New York Times
F D A Approves New H I V Prevention Drug But Not For Everyone The New York Times
Gilead Launches Combination Hiv Pill In Uk And Germany Pmlive
Comments
Post a Comment