- Get link
- X
- Other Apps
- Get link
- X
- Other Apps
Electronic Submissions Gateway Approved Production Transaction Partners Food Facility Registration Module Low Acid Acidified Canned Foods and Account Management. You can select all the products by checking the to p.
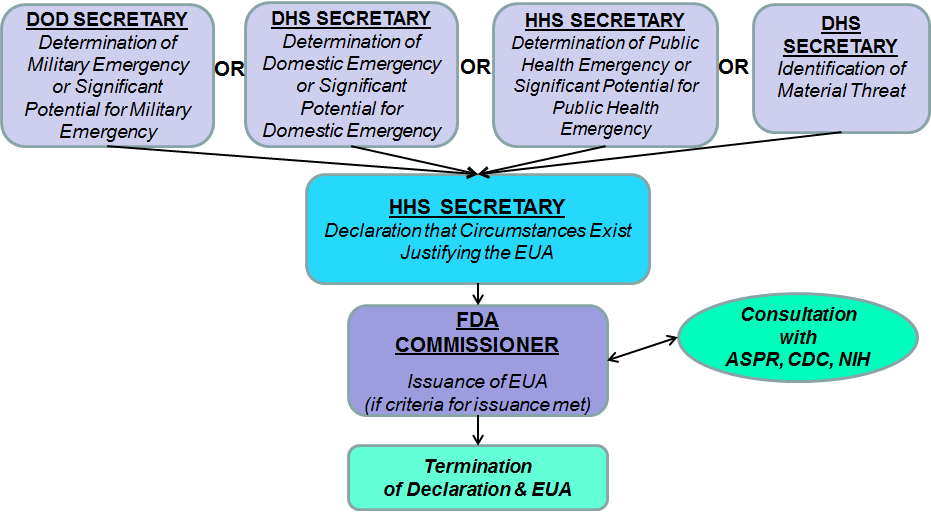
Select the checkbox to choose the products you wish to certify.
Fda certification process. Some companies will pay for an expedited review with the FDA through a process known as an PDUFA Prescription Drug User Fee Act enacted in 1992. FDA registration is not mandatory for cosmetic establishments but can participate in FDA voluntary cosmetic registration program VCRP. FDA batch certification is required for certain color additives.
For more information about how to get FDA certification or FDA approval please contact us at infofdahelpus. FDA Industry Systems FDA Unified Registration and Listing Systems FURLS Technical Help. Some of the product categories require prior approval from the FDA.
FDA registration for cosmetic products is not mandatory FDA does not certify or approve cosmetic products. Certification is a process separate from accreditation. FDA registration is the basic requirement for domestic and foreign establishments that manufacture or market food drug API or medical device in the USA.
The USDA protects consumer options by protecting the organic seal. You can choose to certify all or any of the products Step 11. The requirements to be submitted for the application for Special Certification for COVID-19 Test Kits are the requirements indicated in FDA Memorandum 2020-006 and the copy of the product profile indicating the specificity and sensitivity of the COVID-19 test kit as stipulated in Section III B Paragraph 1 of FDA Memorandum 2021-009.
These guidelines provide minimum requirements that a manufacturer must meet to assure. It is administered by a Certifying Agency FDA or an FDA-approved State Certifying Agency also known as States-as-Certifiers or SACs. The FDA exists to protect public health by assuring the safety efficacy and security of human and veterinary drugs food biological products cosmetics medical devices household chemical substances tobacco and the conduct of clinical trials in the country.
Good manufacturing practices GMP are the practices required in order to conform to the guidelines recommended by agencies that control the authorization and licensing of the manufacture and sale of food and beverages cosmetics pharmaceutical products dietary supplements and medical devices. The FDA also takes action to inspect manufacturing plants where the drug will be made. The drug sponsor must test the drug on multiple species of animals to gather basic information about the compounds safety and efficacy.
The US FDA medical device IVD approval process explained Step 1 Determine the classification of your medical device or in vitro diagnostic IVD device by searching the FDA classification database using relevant search terms or by identifying another predicate device with the same intended use and technology. The organic brand provides consumers with more choices in the marketplace. Apply and Register for License to Operate Certificate of Product Registration and other FDA Authorizations.
1-800-216-7331 or 240-247-8804 730 am-1100 pm. There is no such process or definition of FDA certification in the united states regulatory framework. Certain high-risk colors also require FDA color batch certification of every individual batch.
The registration process varies dependent on the industry but generally involves an annual registration in which organizations are required to list all drugs being manufactured prepared propagated compounded or processed for commercial distribution in the US. What is FDA certification. Color additives may only be used in compliance with their approved uses specifications and restrictions.
Inspection results for FDA registered organizations are available on the FDA website. Flow Chart of FDA Approval Process When a new drug is being developed it needs to go through certain procedures before it can be approved for usesale. FDA Approval of Color Additives FDA approval is required for color additives used in food drugs cosmetics and some medical devices.
A Certificate to Foreign Government is issued for legally marketed devices in the United States that are in compliance with the requirements of the Federal Food Drug and Cosmetic Act FDC. PDUFA allows the FDA to access more resources to quicken the drug approval process. Most of the companies use the term FDA certification for FDA related compliance requirements.
FDA review the sample of each lot of color additive manufactured and certify if it complies with requirement. Organic Certification allows a farm or processing facility to sell label and represent their products as organic.
A Dual Track Drug Approval Process Marginal Revolution
 Fda Approval Process For Anda Download Scientific Diagram
Fda Approval Process For Anda Download Scientific Diagram
 Fda Medical Device Approval Process Step By Step Guide
Fda Medical Device Approval Process Step By Step Guide
 Us Fda Approval Process For Medical Devices
Us Fda Approval Process For Medical Devices
 The Procedure For Import Drug Sfda Registration China Fda Sfda Cfda Moh Moa Aqsiq Cnca Ciq Registration Approval License For Cosmetics Health Food Supplement Medical Device Ivd Drug Infant Milk Powder Dairy Pet Food Disinfectant Etc
The Procedure For Import Drug Sfda Registration China Fda Sfda Cfda Moh Moa Aqsiq Cnca Ciq Registration Approval License For Cosmetics Health Food Supplement Medical Device Ivd Drug Infant Milk Powder Dairy Pet Food Disinfectant Etc
 Exploring Fda Approval Pathways For Medical Devices Massdevice
Exploring Fda Approval Pathways For Medical Devices Massdevice
Fda Update The Fda S New Drug Approval Process Development Premarket Applications
 Fda Approval Process For Anda Download Scientific Diagram
Fda Approval Process For Anda Download Scientific Diagram
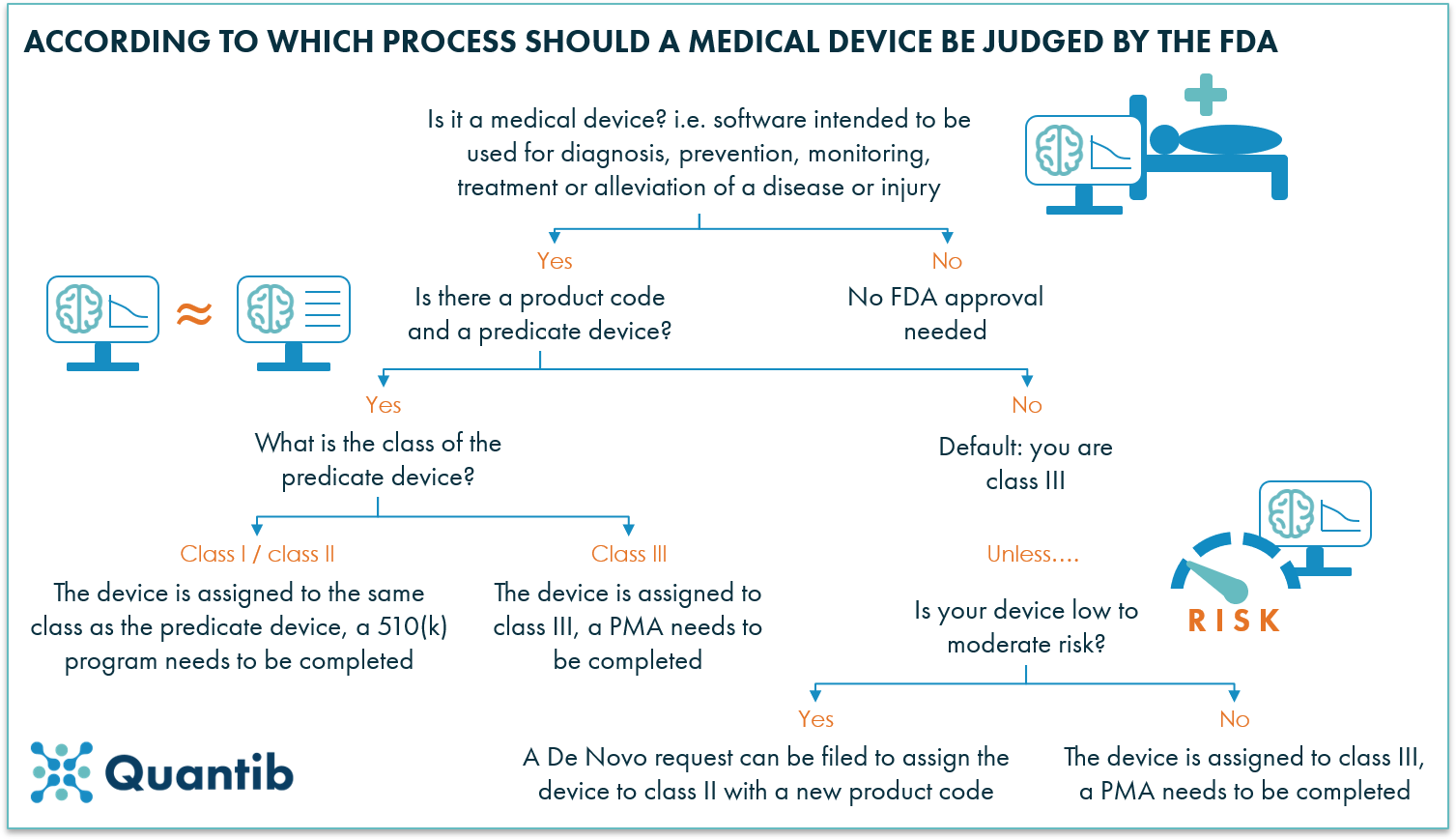
 A 101 Guide To The Fda Regulatory Process For Ai Radiology Software
A 101 Guide To The Fda Regulatory Process For Ai Radiology Software
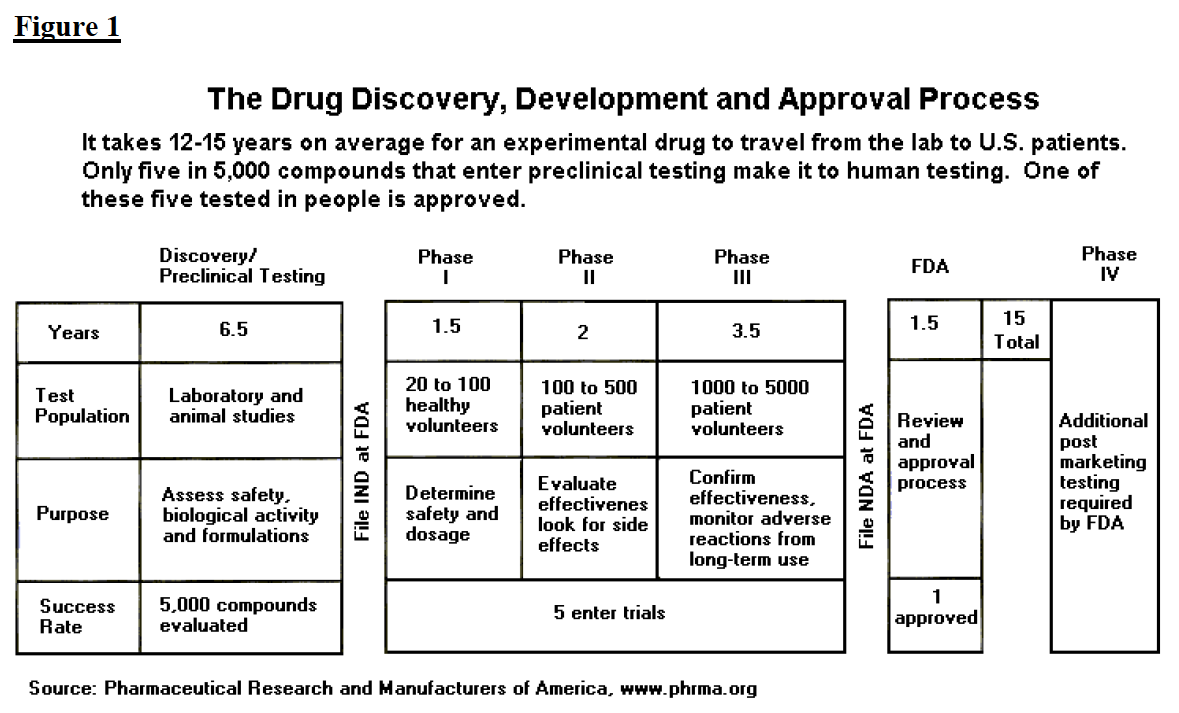 The Drug Development And Approval Process Fdareview Org
The Drug Development And Approval Process Fdareview Org
 How To Get Fda Approval Registrar
How To Get Fda Approval Registrar
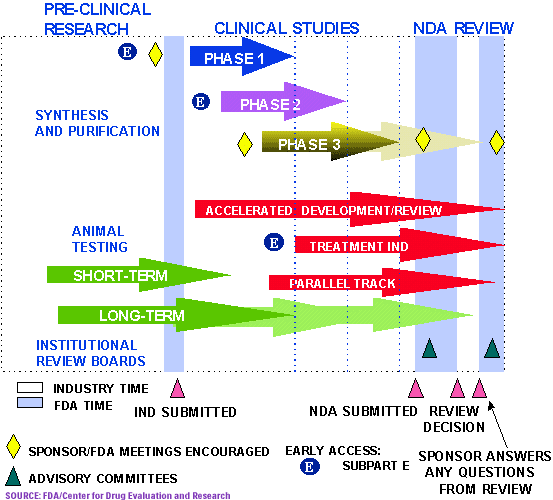 Fda Drug Approval Process Drugs Com
Fda Drug Approval Process Drugs Com
Fda Update The Fda S New Drug Approval Process Development Premarket Applications

Comments
Post a Comment