- Get link
- X
- Other Apps
- Get link
- X
- Other Apps
A 13-valent pneumococcal conjugate vaccine PCV13 Prevnar 13 Pfizer Inc and a 23-valent pneumococcal. Hence if the protein in the urine exceeds 35 grams per day then that person should be vaccinated with the Prevnar 13.
 Pneumococcal Disease Prevention Through Vaccination Cdc
Pneumococcal Disease Prevention Through Vaccination Cdc
Prevenar 13 kan ges tillsammans med andra barnvacciner men på ett annat vaccinationsställe.
Cdc prevnar 13. Prevnar 13 Pneumococcal 13-valent Conjugate Vaccine Diphtheria CRM197 Protein is a vaccine approved for adults 18 years of age and older for the. Prevenar 13 kan vid vaccination av vuxna i åldrarna 50 år eller äldre ges samtidigt som det trivalenta inaktiverade influensavaccinet. 128915 in adults aged 65 and older1 Importantly Prevnar 13.
Prevnar 13 vaccine is used to prevent infection caused by pneumococcal bacteria. PFE announced today that results from a study analyzing real-world effectiveness data found that Prevnar 13 pneumococcal 13-valent conjugate vaccine diphtheria CRM197 Protein reduced the risk of hospitalization from vaccine-type pneumococcal community-acquired pneumonia CAP by 73 95 CI. It is also recommended for children and adults 2 to 64 years of age with certain health conditions and for all adults 65 years of age and older.
Your doctor can give you. Infants and young children usually need 4 doses of pneumococcal conjugate vaccine at 2 4 6 and 12 15 months of age. CDC states that it is acceptable to use out-of-date.
Prevenar 13 får inte blandas med andra vacciner i samma spruta. Prevnar 13 was approved for use in the European Union in December 2009. Active immunization for the prevention of pneumonia and invasive disease caused by S.
Prevnar 13 is indicated for active immunization for the prevention of invasive disease caused by 13 strains of Streptococcus pneumoniae 1 3 4 5 6A 6B 7F 9V 14 18C 19A 19F and 23F. In adults 18 years of age and older Prevnar 13 is indicated for. PCV13 is routinely given to children at 2 4 6 and 1215 months of age.
At that time IAC will provide translations in Spanish Arabic Burmese Chinese Simplified and Traditional French Russian Somali and Vietnamese. Pneumococcal conjugate PCV13 VIS. The interim VIS issued by CDC on 103019 will be replaced by a final version that is expected in 2021.
When the decision to administer Prevnar 13 is made the information below represents the CDCs ACIP recommendations to complete the pneumococcal vaccination. PFE issued the following statement in response to todays discussion by the Centers for Disease Control and Preventions CDC Advisory Committee on Immunization Practices ACIP regarding the use of Prevnar 13 Pneumococcal 13-valent Conjugate Vaccine Diphtheria CRM 197 Protein in. They are in the process of being adopted by physicians and insurance coverage.
Pneumococcal conjugate vaccine called PCV13 protects against 13 types of pneumococcal bacteria. In some cases a child might need fewer than 4 doses to complete PCV13 vaccination. PCV13 protects against 13 types of bacteria that cause pneumococcal disease.
THE CDCS ACIP RECOMMENDS PREVNAR 13 FOR IMMUNOCOMPROMISED ADULTS AGED 19 AND FOR IMMUNOCOMPETENT ADULTS AGED 65 BASED ON SHARED CLINICAL DECISION-MAKING1. Pneumococcal conjugate vaccine use in children has led to sharp declines in pneumococcal disease among adults and children. A dose of PCV13 vaccine is also recommended.
Infants and young children usually need 4 doses of pneumococcal conjugate vaccine at 2 4 6 and 1215 months of age. Two pneumococcal vaccines are currently licensed for use in adults in the United States. The CDCs ACIP recommends Prevnar 13 for immunocompetent adults aged 65 and older based on shared clinical decision-making 1 Pneumovax 23 is recommended for all adults 65 and older.
PCV13 protects against 13 types of bacteria that cause pneumococcal disease. Wednesday February 22 2012 - 534am EST Pfizer Inc. Prevnar 13 is a vaccine approved for use in children 6 weeks through 5 years of age prior to the 6 th birthday.
Prevnar 13 contains 13 different types of pneumococcal bacteria. Prevnar 13 Pneumococcal 13-valent Conjugate Vaccine Diphtheria CRM 197 Protein is a vaccine approved for adults 18 years of age and older for the prevention of pneumococcal pneumonia and invasive disease caused by 13 Streptococcus pneumoniae strains 1 3. Prevnar 13 PCV13 is produced by Pfizer formerly Wyeth and replaced Prevnar.
Announced that the Centers for Disease Control and Preventions CDC Advisory Committee on Immunization Practices ACIP voted to recommend Prevnar 13 Pneumococcal 13-valent. In some cases a child might need fewer than 4 doses to complete PCV13 vaccination. Pneumococcal disease is a serious infection caused by a bacteria.
It is a tridecavalent vaccine it contains thirteen serotypes of pneumococcus 1 3 4 5 6A 6B 7F 9V 14 18C 19A 19F and 23F which are conjugated to diphtheria carrier protein. These are new guidelines just adopted by the CDC and suggested by the Advisory Committee for Immunization Practices ACIP.
 Safety Info Prevnar 13 Pneumococcal 13 Valent Conjugate Vaccine Diphtheria Crm197 Protein Cdc S Acip Adult Recommendations For Prevnar 13
Safety Info Prevnar 13 Pneumococcal 13 Valent Conjugate Vaccine Diphtheria Crm197 Protein Cdc S Acip Adult Recommendations For Prevnar 13
 Pneumococcal Vaccine Recommendations Cdc
Pneumococcal Vaccine Recommendations Cdc
Https Cdn Ymaws Com Www Louisianapharmacists Com Resource Collection Dccf5a21 Deef 48a0 9ebb 4a08818eed73 September 2018 Psa Pdf
 Pcv13 Pneumococcal Conjugate Vaccine For Adults For Providers Cdc
Pcv13 Pneumococcal Conjugate Vaccine For Adults For Providers Cdc
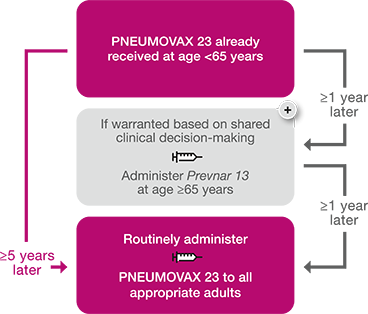 Pneumococcal Vaccine For Ages 65 Cdc Recommendations
Pneumococcal Vaccine For Ages 65 Cdc Recommendations
 Administering Pneumococcal Vaccine For Providers Cdc
Administering Pneumococcal Vaccine For Providers Cdc
 Pneumococcal Vaccination In Adults Uptodate
Pneumococcal Vaccination In Adults Uptodate
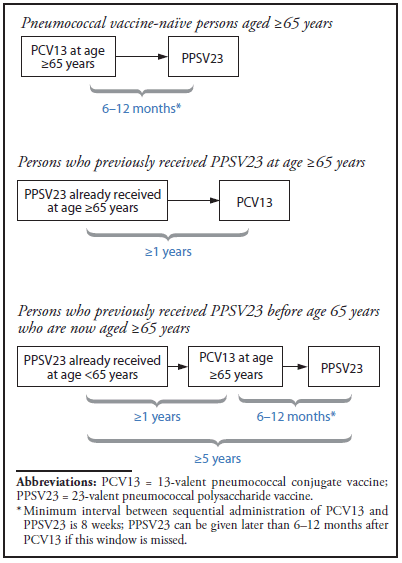 Use Of 13 Valent Pneumococcal Conjugate Vaccine And 23 Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged 65 Years Recommendations Of The Advisory Committee On Immunization Practices Acip
Use Of 13 Valent Pneumococcal Conjugate Vaccine And 23 Valent Pneumococcal Polysaccharide Vaccine Among Adults Aged 65 Years Recommendations Of The Advisory Committee On Immunization Practices Acip
 Safety Info Prevnar 13 Pneumococcal 13 Valent Conjugate Vaccine Diphtheria Crm197 Protein Cdc S Acip Adult Recommendations For Prevnar 13
Safety Info Prevnar 13 Pneumococcal 13 Valent Conjugate Vaccine Diphtheria Crm197 Protein Cdc S Acip Adult Recommendations For Prevnar 13
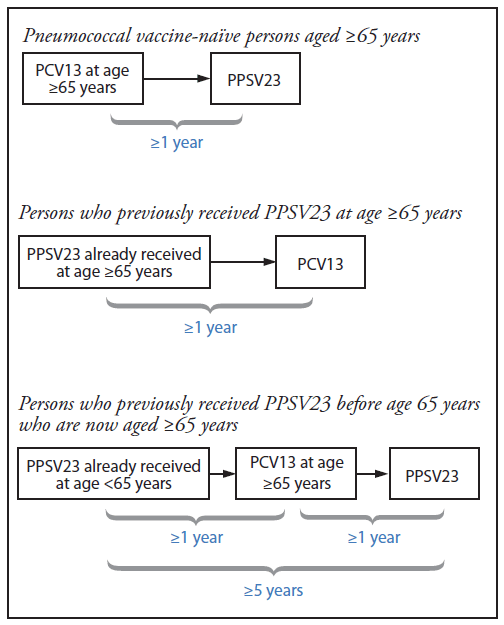 Intervals Between Pcv13 And Ppsv23 Vaccines Recommendations Of The Advisory Committee On Immunization Practices Acip
Intervals Between Pcv13 And Ppsv23 Vaccines Recommendations Of The Advisory Committee On Immunization Practices Acip
 Safety Info Prevnar 13 Pneumococcal 13 Valent Conjugate Vaccine Diphtheria Crm197 Protein Cdc S Acip Adult Recommendations For Prevnar 13
Safety Info Prevnar 13 Pneumococcal 13 Valent Conjugate Vaccine Diphtheria Crm197 Protein Cdc S Acip Adult Recommendations For Prevnar 13
 Pneumococcal Vaccine For Ages 65 Cdc Recommendations
Pneumococcal Vaccine For Ages 65 Cdc Recommendations
 Safety Info Prevnar 13 Pneumococcal 13 Valent Conjugate Vaccine Diphtheria Crm197 Protein Cdc S Acip Adult Recommendations For Prevnar 13
Safety Info Prevnar 13 Pneumococcal 13 Valent Conjugate Vaccine Diphtheria Crm197 Protein Cdc S Acip Adult Recommendations For Prevnar 13
Comments
Post a Comment